CK is a dimer composed of two different subunits, B and M. The three isoenzymes are designated MM, MB and BB. The CK isoenzymes are resolved by electrophoresis but many hospital laboratories conduct CK isoenzyme analysis by a simple procedure involving the use of an antibody against the M subunit which inhibits M activity while the activity of the B subunit is retained.
The BB isoenzyme is present in brain and intestinal smooth muscle, but the BB isoenzyme is rarely present in serum, even in cases of nervous system or intestinal disease. The major CK isoenzyme of both skeletal muscle and myocardium is MM, and both tissues contain the MB isoenzyme. The ratio of MB/MM is, however, different for each tissue. The skeletal muscle content of MB is less than 1% of total CPK activity, whereas the myocardial content of MB is about 25-50% of total CK activity. Normal serum activity is almost entirely from the MM isoenzyme and the MB content of normal serum is usually undetectable. Degenerative muscle disease or crushing muscle injury results in elevated total serum CK activity, but the MB isoenzyme content is less than 5%. Serum MB activity greater than 5% of total is considered diagnostic for MI.
CK and/or LDH isoenzyme determinations are useful when there is question about the tissue source of elevated enzyme activity.
Correspondence Between CK and LDH Isoenzyme Findings
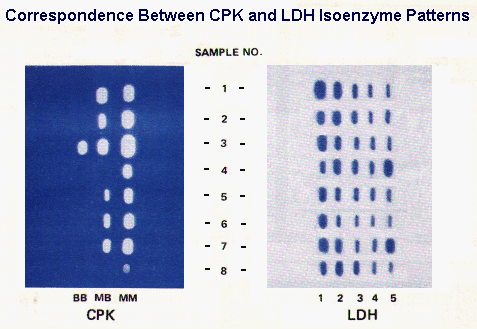 The correspondence between CK and LDH isoenzyme findings in different conditions is illustrated in the figure to the right.
The correspondence between CK and LDH isoenzyme findings in different conditions is illustrated in the figure to the right.
Sample #3 represent results for a control specimen to assure that the technique is working properly and that all bands present are detected.
Sample #8 results are from a normal specimen.
Sample# 1 results are from an MI patient. The specimen was collected at a time when the activity of both LDH and CK were elevated Note the LDH flip and the high relative activity of the MB isoenzyme.
Sample# 2 results are from an MI patient who experienced chest pain only several hours previously. Total CK is significantly elevated with a high relative MB isoenzyme activity.
Sample# 4 results are from a patient with liver disease. Although the LDH isoenzyme pattern is indistinguishable from muscle disease or injury, the absence of at least a trace of CK-MB isoenzyme is inconsistent with the muscle CPK isoenzyme distribution as is the apparently normal total activity.
Sample# 5 results are from an MI patient; the specimen was collected about 2 days post MI so that CK has almost returned to normal activity and the LDH flip is definite.
Sample# 6 results are from an MI patient with the specimen collected about the 1st day post MI; CK activity is definitely elevated with a high relative MB isoenzyme activity and the LDH flip is evident.
Sample# 7 results are from an MI patient with complications of heart failure and passive liver congestion or the patient was involved in an accident as a consequence of the MI, and suffered a crushing muscle injury.
 Myocardial infarction results in death of myocardial cells served by the effected artery after about 30 minutes of anoxia. The acute inflammatory response, as a consequence of cell death, results in fever, leukocytosis, and increased concentrations of acute phase reactant proteins in the circulation (determined in the clinical laboratory most commonly by the ESR but better by measurement of C-reactive protein). An earlier and more specific consequence of myocardial death is the liberation of intracellular contents and their appearance in the circulation several hours later following diffusion through the interstitium into patent vessels and lymph ducts. Intracellular markers, routinely determined by laboratory testing, are certain enzymes present at high activity in the tissue. The enzymes routinely measured in the clinical laboratory for the purpose of diagnosing and monitoring myocardial infarction include creatine kinase (CK), aspartate amino transferase (sGOT or AST), and lactate dehydrogenase (LDH). These enzymes are present in sufficiently high content in myocardial tissue so that the death of a relatively small amount of tissue results in a substantial increase in measured enzyme activity in serum.
Myocardial infarction results in death of myocardial cells served by the effected artery after about 30 minutes of anoxia. The acute inflammatory response, as a consequence of cell death, results in fever, leukocytosis, and increased concentrations of acute phase reactant proteins in the circulation (determined in the clinical laboratory most commonly by the ESR but better by measurement of C-reactive protein). An earlier and more specific consequence of myocardial death is the liberation of intracellular contents and their appearance in the circulation several hours later following diffusion through the interstitium into patent vessels and lymph ducts. Intracellular markers, routinely determined by laboratory testing, are certain enzymes present at high activity in the tissue. The enzymes routinely measured in the clinical laboratory for the purpose of diagnosing and monitoring myocardial infarction include creatine kinase (CK), aspartate amino transferase (sGOT or AST), and lactate dehydrogenase (LDH). These enzymes are present in sufficiently high content in myocardial tissue so that the death of a relatively small amount of tissue results in a substantial increase in measured enzyme activity in serum.
 The change in activity of each enzyme in serum following myocardial infarction exhibits a characteristic time course as illustrated in the figure to the right:
The change in activity of each enzyme in serum following myocardial infarction exhibits a characteristic time course as illustrated in the figure to the right:
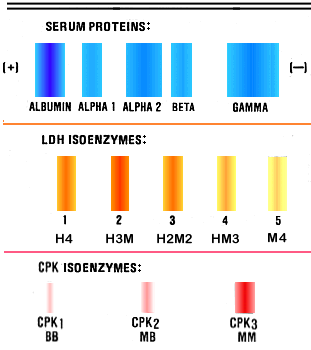 LDH is a tetramer composed of two different subunits, M and H. Five different isoenzymes are possible: M4, M3H, M2H2, MH3, and H4, and all five are present in different tissue in different relative amounts. The five different isoenzymes are resolved by electrophoresis and determination of the LDH isoenzyme distribution by electrophoresis is a commonly performed clinical laboratory test. The predominant LDH isoenzyme in liver and skeletal muscle is M4 (LDH-5) and the predominant cardiac isoenzyme is H4 (LDH-1). The LDH isoenzyme distribution in normal serum is characterized by the major band being MH3 (LDH-2).
When total LDH activity is increased from muscle or liver disease, the LDH-5 band becomes dominant. Increased LDH activity from MI is characterized by a predominance of the LDH-1 band. The change from the dominance of the LDH-2 band in normal serum to the dominance of the LDH-1 band is called the "LDH flip" and its occurrence is considered diagnostic for MI. However, the major LDH isoenzyme in erythrocytes is LDH-1 so that hemolysis, either intravascular or in the collected blood specimen, may also cause the LDH flip.
LDH is a tetramer composed of two different subunits, M and H. Five different isoenzymes are possible: M4, M3H, M2H2, MH3, and H4, and all five are present in different tissue in different relative amounts. The five different isoenzymes are resolved by electrophoresis and determination of the LDH isoenzyme distribution by electrophoresis is a commonly performed clinical laboratory test. The predominant LDH isoenzyme in liver and skeletal muscle is M4 (LDH-5) and the predominant cardiac isoenzyme is H4 (LDH-1). The LDH isoenzyme distribution in normal serum is characterized by the major band being MH3 (LDH-2).
When total LDH activity is increased from muscle or liver disease, the LDH-5 band becomes dominant. Increased LDH activity from MI is characterized by a predominance of the LDH-1 band. The change from the dominance of the LDH-2 band in normal serum to the dominance of the LDH-1 band is called the "LDH flip" and its occurrence is considered diagnostic for MI. However, the major LDH isoenzyme in erythrocytes is LDH-1 so that hemolysis, either intravascular or in the collected blood specimen, may also cause the LDH flip.
 The correspondence between CK and LDH isoenzyme findings in different conditions is illustrated in the figure to the right.
The correspondence between CK and LDH isoenzyme findings in different conditions is illustrated in the figure to the right.
 Other intracellular proteins are potentially useful markers for MI. The time course of three, recently proposed, markers are shown, in comparison to that of CK-MB, in the figure to the right.
Other intracellular proteins are potentially useful markers for MI. The time course of three, recently proposed, markers are shown, in comparison to that of CK-MB, in the figure to the right.