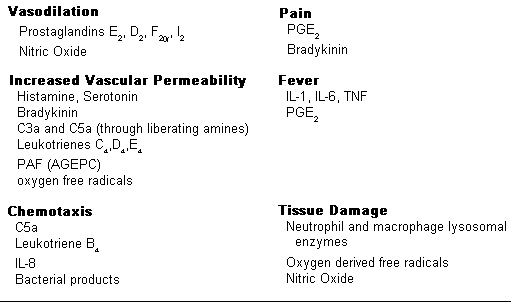
 The important substances mediating the processes of acute inflammation are displayed to the right:
The important substances mediating the processes of acute inflammation are displayed to the right:
I. The important mediators of acute inflammation
 The important substances mediating the processes of acute inflammation are displayed to the right:
The important substances mediating the processes of acute inflammation are displayed to the right:
II. Mediator Systems
The mediators of acute inflammation are classified into systems based on their source and/or chemical composition.
Aspirin and other NSAIDs inhibit cyclooxygenase but not lipoxygenase. Anti-inflammatory steroids stabilize membranes and prevent phospholipid hydrolysis thereby inhibiting both pathways.
The significance of the effects of reactive oxygen metabolites during inflammation depends upon the relative rates of formation and of elimination by the antioxidant protective mechanisms: transferrin and ceruloplasmin, superoxide dismutase, catalase and glutathione peroxidase.
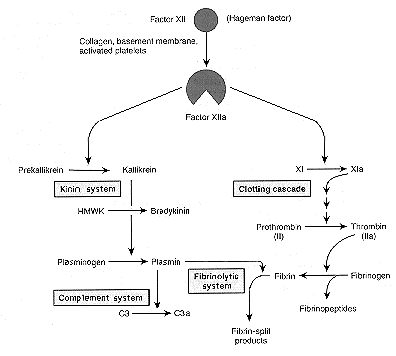
Clotting does not occur until later in the acute inflammatory response unless injury is severe or vasculature is damaged. Early clotting is apparently inhibited by heparin released from mast cells and much fibrinogen (MW = 340,000) probably does not enter the exudate unless vascular permeability is greatly increased from more severe injury. Mediators of the clotting/fibrinolysis system include:
- converts fibrinogen to fibrin and fibrinopeptides - induce increased vascular permeability - chemotactic for leukocytes - activates complement components C3 and C5 - splits fibrin to fibrin split products - may induce increased vascular permeability
 The ultimate objective of the complement system is the formation of the terminal C5-9 assemblage on plasma membranes resulting in the production of large pores effecting cell lysis. Other split products generated as the sequence proceeds are important inflammatory mediators:
The ultimate objective of the complement system is the formation of the terminal C5-9 assemblage on plasma membranes resulting in the production of large pores effecting cell lysis. Other split products generated as the sequence proceeds are important inflammatory mediators:
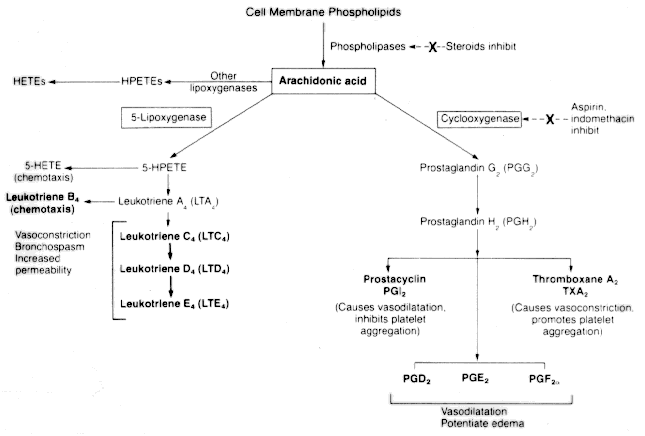
Different cells have different enzyme systems and synthesize different eicosinoids. Leukocytes, mast cells and platelets synthesize leukotrienes. Platelets also synthesize thromboxane A2 (TXA2). Vascular endothelia synthesize prostacyclin (PGI2). PGD2 is the principal PG synthesized by mast cells. PGD2 and PGE2 are synthesized by a number of different cells.
Prostaglandins:
Leukotrienes:
AGEPC also stimulates target cells to synthesize eicosinoids and augments their effects.
� sleepiness
� decreased appetite
� hemodynamic effects - hypotension, decreased vascular resistance, increased heart rate
� leukocytosis
� induction of hepatic synthesis of acute phase proteins (fibrinogen, complement components, C reactive protein)
� increased adhesion of leukocytes to endothelium
� increased elaboration of PGI2 and AGEPC
� increased thrombogenicity of the endothelial surface
� increased collagenase and protease synthesis
� increased synthesis of PGE2
The effects of the released lysosomal consituents are prevented from spreading beyond the site of inflammation by the presence in the circulation of inhibitors including alpha-1-antitrypsin and alpha-2-macroglobulin.