Acute Respiratory Acidosis
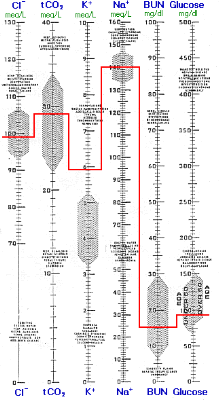
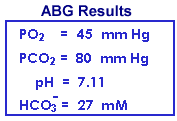 Respiration may diminish in an acute manner from a variety of etiologies, including overdose of CNS depressant drugs, crushing chest injuries, atelectesis, etc. Decreased respiration develops over a time period of hours or less; insufficient time for significant renal compensation. Even if PCO2 increases by a factor of 2 or 3, the increase in total CO2 (from the 2 - 4 mM increase in dissolved CO2) determined by the chemistry instrument is barely noticeable. The PCO2 and pH results from the blood gas instrument are far more revealing. Blood gas results are useful for evaluating respiratory acid/base disturbances, but are unnecessary for evaluating metabolic acid/base disturbances in which cases more dramatic total CO2 changes are generally readily evident.
Respiration may diminish in an acute manner from a variety of etiologies, including overdose of CNS depressant drugs, crushing chest injuries, atelectesis, etc. Decreased respiration develops over a time period of hours or less; insufficient time for significant renal compensation. Even if PCO2 increases by a factor of 2 or 3, the increase in total CO2 (from the 2 - 4 mM increase in dissolved CO2) determined by the chemistry instrument is barely noticeable. The PCO2 and pH results from the blood gas instrument are far more revealing. Blood gas results are useful for evaluating respiratory acid/base disturbances, but are unnecessary for evaluating metabolic acid/base disturbances in which cases more dramatic total CO2 changes are generally readily evident.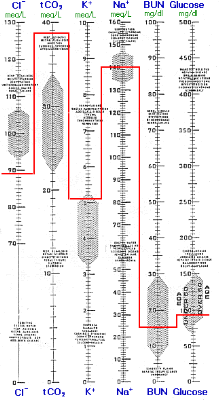
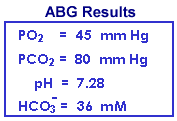 Renal compensation is nearly complete following several days of diminished respiration or in cases of chronic lung disease (e.g., emphysema). The high-normal or slightly increased K+ and the increased total CO2 is consistent only with compensated respiratory acidosis and is inconsistent with metabolic alkalosis. Nevertheless, the blood gas results are more revealing and the PO2 and PCO2 are routinely used to monitor patients' progress. Note that the increased HCO3- from renal compensation resulted in an increase of 0.11 pH unit compared to the previous analogous example of acute respiratory acidosis.
Renal compensation is nearly complete following several days of diminished respiration or in cases of chronic lung disease (e.g., emphysema). The high-normal or slightly increased K+ and the increased total CO2 is consistent only with compensated respiratory acidosis and is inconsistent with metabolic alkalosis. Nevertheless, the blood gas results are more revealing and the PO2 and PCO2 are routinely used to monitor patients' progress. Note that the increased HCO3- from renal compensation resulted in an increase of 0.11 pH unit compared to the previous analogous example of acute respiratory acidosis.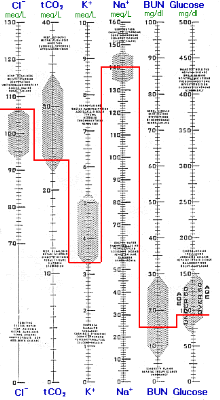
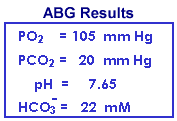 Increased respiration occurs briefly because of stress or may occur for more prolonged periods of time in psychotics. Mountain vacationers unaccustomed to the low PO2 of high altitudes are also forced to respire more rapidly because of stimulation by the peripheral O2 sensors. The half-time for renal compensation of respiratory alkalosis is about 8 hours. Renal compensation is insignificant during the first few hours and the chemistry results are not very remarkable except for the slightly decreased K+ along with the low-normal total CO2 only suggesting the condition. Blood gas results are far more informative.
Increased respiration occurs briefly because of stress or may occur for more prolonged periods of time in psychotics. Mountain vacationers unaccustomed to the low PO2 of high altitudes are also forced to respire more rapidly because of stimulation by the peripheral O2 sensors. The half-time for renal compensation of respiratory alkalosis is about 8 hours. Renal compensation is insignificant during the first few hours and the chemistry results are not very remarkable except for the slightly decreased K+ along with the low-normal total CO2 only suggesting the condition. Blood gas results are far more informative.
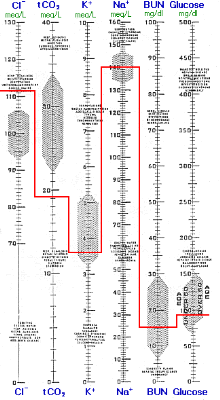
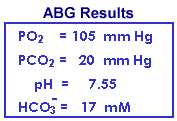 Renal compensation for respiratory alkalosis is nearly complete after the first day of increased respiration. The low-normal K+ and the decreased total CO2 is consistent with compensated respiratory alkalosis, but the blood gas results are more definitive.
Renal compensation for respiratory alkalosis is nearly complete after the first day of increased respiration. The low-normal K+ and the decreased total CO2 is consistent with compensated respiratory alkalosis, but the blood gas results are more definitive.