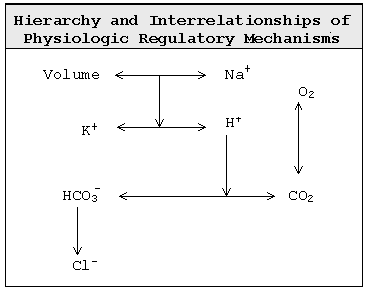
Regulation of fluid volume, electrolyte concentrations and acid/base balance are interrelated in a hierarchical manner. Fluid volume and osmolality are regulated interdependently and with highest priority.
Sodium concentration is regulated
by way of renal sensing of perfusion pressure which in most cases reflects fluid volume status. Low pressure results in renal renin elaboration with the consequent formation of angiotensin-II and decreased renal blood flow, and thus decreased GFR, and also stimulation of adrenal cortical elaboration of aldosterone thereby increasing renal Na+ reabsorption. Renin release, and subsequent effects, is inhibited by elevated renal perfusion pressure. Although angiotensin-II is the major factor stimulating adrenal cortical aldosterone elaboration, hyperkalemia also stimulates, but to a lesser extent.
Plasma K+ and H+ regulation are interrelated and are subservient to Na+ regulation. Potassium is an abundant nutrient and a mechanism for its regulation (other than intracellular sequestration with the Na+ /K+ ATPase membrane pump and renal elimination in proportion to intracellular concentration) has not evolved. Aldosterone stimulated Na+ reabsorption causes increased K+ and H+ secretion and consequent hypokalemia and alkalosis. When aldosterone stimulated Na+ reabsorption is inhibited, then K+ and H+ secretion are minimal so that hyperkalemia and acidosis result.
Bicarbonate concentration is a reflection of acid/base status.
Cl- concentration is not directly regulated but changes passively, as demanded for maintenance of electroneutrality, with changes in concentration of other ions.
Fluid volume is regulated by way of pituitary sensing of osmolality. Hyperosmolality stimulates thirst and causes pituitary elaboration of ADH which stimulates renal water reabsorption. (Urea reabsorption accompanies ADH stimulated water reabsorption in the distal nephron.) The extent of water reabsorption is limited by the quantity of solutes in tubule fluid, the concentration of which cannot exceed the osmolality of the hypertonic medulla, about 1400 mOsM. Because the daily excrecreted solute load is about 500-700 mOsmoles, minimum urine volume is about 300-500 ml/day.
The kidneys sense hypovolemia as decreased perfusion pressure and respond by elaborating renin with the consequent formation of angiotensin-II causing decreased renal blood flow and, thus, decreased GFR, and also stimulating the adrenal cortex to synthesize and release aldosterone, thereby increasing renal Na+ reabsorption.
A hypovolemic state is thus generally revealed by a high urine osmolality (> 900 mOsM), a low urine Na+ concentration (generally < 25 mEq/L; a better gauge of the extent of Na+ reabsorption is the fraction of filtered sodium excreted which is less than 1 % in hypovolemic cases), increased serum urea and creatinine concentrations, and an increased serum urea/creatinine ratio (the normal ratio is about 15).
 Aldosterone stimulates Na+ reabsorption in the collecting tubules and is accompanied by counter secretion of K+ and H+, rather than by coreabsorption of anions as in the proximal tubule.
Aldosterone stimulates Na+ reabsorption in the collecting tubules and is accompanied by counter secretion of K+ and H+, rather than by coreabsorption of anions as in the proximal tubule.
Sodium excess stimulates thirst and water reabsorption resulting in hypervolemia and hypertension rather than in frank hypernatremia. Hypervolemia diminishes Na+ reabsorption and the consequent decreased K+ and/or H+ secretion causes mild hyperkalemia and acidemia.
Sodium deficit results in diminished thirst and water reabsorption and thereby hypovolemia rather than hyponatremia. The consequent aldosterone stimulated renal Na+ reabsorption and increased K+ and H+ secretion causes hypokalemia and alkalemia.
Osmolality (Na+) and fluid volume regulation has priority over H+ and K+ regulation.
In acidosis, renal H+ secretion is increased so that there is a corresponding decrease in K+ secretion contributing to hyperkalemia. In alkalosis there is diminished H+ secretion and so increased renal K+ secretion contributing to hypokalemia. On the other hand, hyperkalemia diminishes H+ secretion and contributes to acidemia; and hypokalemia causes increased H+ secretion and alkalemia. Another factor contributing to the reciprocal relationship between circulating K+ and H+ concentrations is the "K+/H+ shift". K+ enters and H+ exits cells in hyperkalemia, and vice versa in acidemia. K+ exits and H+ enters cells in hypokalemia, and vice versa in alkalemia. The acidemia caused by hyperkalemia is anamolous since it is associated with a high intracellular and urinary pH. The alkalemia caused by hypokalemia is similarly anamolous.
Anion Gap




