-
Nephrotic Syndrome
(from increased glomerular permeability to proteins)-
characteristic features:
- proteinuria > 3-4g/day; the protein loss may be
"selective" - predominately albumin lost - or
"nonselective" (large as well as small proteins lost) - hypoproteinemia and consequent edema (the protein synthesizing capacity of the liver is exceeded when protein loss is greater than 3-4g/day)
- hyperlipidemia and elevated alpha-2 macroglobulin (from generally increased hepatic protein synthesis and retention of very large proteins)
- proteinuria > 3-4g/day; the protein loss may be
-
characteristic disease entities:
-
minimal change disease (lipoid nephrosis)
- selective proteinuria -
membranous glomerulonephritis
- nonselective proteinuria
-
minimal change disease (lipoid nephrosis)
- Nephritic Syndrome
(from inflammatory injury to glomeruli)-
characteristic features:
- azotemia
- decreased concentrating ability
- hematuria
- proteinuria (< 2g/day)
- red cell casts
-
characteristic disease entities:
- acute post streptococcal glomerulonephritis
- rapidly progressive glomerulonephritis
- Tubulointersitial Disease
(from inflammation affecting tubules)-
characteristic features
- cloudy urine from high WBC count
- white cell casts
- proteinuria (< 1g/day)
- decreased concentrating ability
- azotemia
-
characteristic disease entities
- pyelonephritis
- analgesic induced interstitial nephritis
- Chronic Renal Failure
- develops insidiously over a time course of years from nephritic or tubulointersitial disease.
- may first be noticed from proteinuria or azotemia or in later stages from uremic symptoms
- important to detect early when disease is treatable
- Acute Renal Failure
(from anoxia or nephrotoxins)-
characteristic features
- sudden oliguria
- rapidly developing azotemia
- broad waxy casts
- loss of concentrating ability
- diminished sodium reabsorption
-
characteristic disease entities
- acute tubular necrosis
- diffuse cortical necrosis
- important to distinquish from cases of prerenal azotemia
Progressive Impairment of Individual Functions
A. Decrease in Glomerular Filtration Rate and Progression of Azotemia: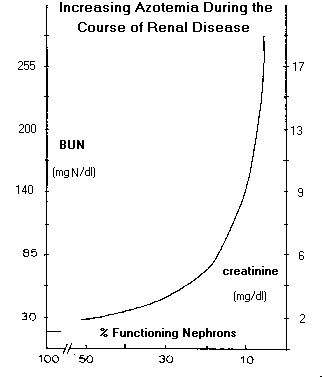 As glomerular filtration rate decreases during the course of renal disease, substances, which are normally excreted, primarily by filtration, accumulate and serum concentrations increase. In the past, these substances were determined collectively as nonprotein nitrogen (NPN) components (thus, the term "azotemia") and included urea, creatinine and uric acid. The NPN assay is no longer conducted and the individual components are currently measured independently. Uric acid is excreted primarily by active secretion for which there is sufficient excess capacity so that hyperuricemia does not develop until the renal failure stage. Urea and creatinine, however, are excreted almost exclusively by filtration so that their serum concentrations increase in proportion to decreased GFR. For example, serum concentrations of urea and creatinine increase by a factor of two when GFR decreases by a factor of two, etc. Serum urea concentration is sufficiently great so that increases cause an increase in serum osmolality. Azotemia (increased serum concentrations of urea and creatinine) is not reliably found until GFR has decreased by about 50%.
As glomerular filtration rate decreases during the course of renal disease, substances, which are normally excreted, primarily by filtration, accumulate and serum concentrations increase. In the past, these substances were determined collectively as nonprotein nitrogen (NPN) components (thus, the term "azotemia") and included urea, creatinine and uric acid. The NPN assay is no longer conducted and the individual components are currently measured independently. Uric acid is excreted primarily by active secretion for which there is sufficient excess capacity so that hyperuricemia does not develop until the renal failure stage. Urea and creatinine, however, are excreted almost exclusively by filtration so that their serum concentrations increase in proportion to decreased GFR. For example, serum concentrations of urea and creatinine increase by a factor of two when GFR decreases by a factor of two, etc. Serum urea concentration is sufficiently great so that increases cause an increase in serum osmolality. Azotemia (increased serum concentrations of urea and creatinine) is not reliably found until GFR has decreased by about 50%.
____________________________________________________________ B. Diminished Ability to Regulate Fluid Volume:
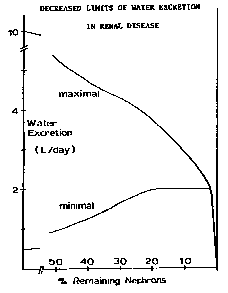 Regulation of fluid volume requires the ability to retain water and excrete a concentrated urine during periods of low water intake and to excrete excess water when fluid intake is high. Maximum water excretion is 6 - 7% of GFR and is normally about 10 L/day. This much water is excreted by patients with diabetes insipidus (ADH lack). As GFR decreases, so does the maximum water excretion rate. The ability to retain water depends upon concentrating ability which diminishes as the number of functioning nephrons decreases. Normal concentrating ability allows excretion of urine having an osmolality 3 - 4 times that of plasma, and a minimum urine volume of about 300 - 500 ml/day for a usual solute load to be excreted of about 600 mosmoles. Concentrating ability is completely lost at the renal failure stage and maximum urine osmolality is the same as plasma osmolality. Minimum urine volume is then about 2 L/day or greater if the osmolar load is greater than usual. Adequate volume regulation can be maintained during the course of renal disease only if water intake is between the limits imposed by the minimum and maximum urine excretion rates of the functionally impaired kidneys. If water intake is less than about 2 L/day (plus the amount required to compensate for insensible water loss) then dehydration results. If water intake is greater than the maximum amount which can be excreted, then hypervolemia and hypertension result. Because of the usual 300-500 ml capacity of the bladder, nocturia generally begins during the renal insufficiency stage and persists during the stages of renal failure and uremia as polyuria (urine volume > 2 L/day) develops. During end stage, water excretion is sufficiently diminished so that oliguria ( < 400 ml urine/day) occurs. Frank anuria ( < 50 ml/day) is rare.
Regulation of fluid volume requires the ability to retain water and excrete a concentrated urine during periods of low water intake and to excrete excess water when fluid intake is high. Maximum water excretion is 6 - 7% of GFR and is normally about 10 L/day. This much water is excreted by patients with diabetes insipidus (ADH lack). As GFR decreases, so does the maximum water excretion rate. The ability to retain water depends upon concentrating ability which diminishes as the number of functioning nephrons decreases. Normal concentrating ability allows excretion of urine having an osmolality 3 - 4 times that of plasma, and a minimum urine volume of about 300 - 500 ml/day for a usual solute load to be excreted of about 600 mosmoles. Concentrating ability is completely lost at the renal failure stage and maximum urine osmolality is the same as plasma osmolality. Minimum urine volume is then about 2 L/day or greater if the osmolar load is greater than usual. Adequate volume regulation can be maintained during the course of renal disease only if water intake is between the limits imposed by the minimum and maximum urine excretion rates of the functionally impaired kidneys. If water intake is less than about 2 L/day (plus the amount required to compensate for insensible water loss) then dehydration results. If water intake is greater than the maximum amount which can be excreted, then hypervolemia and hypertension result. Because of the usual 300-500 ml capacity of the bladder, nocturia generally begins during the renal insufficiency stage and persists during the stages of renal failure and uremia as polyuria (urine volume > 2 L/day) develops. During end stage, water excretion is sufficiently diminished so that oliguria ( < 400 ml urine/day) occurs. Frank anuria ( < 50 ml/day) is rare.
____________________________________________________________ C. Decreased Excretion of Acid, Phosphate, Urate and Other Organic Anions:
The normal kidneys can excrete 3 - 5 times as much acid (as NH4+) as usually required and, therefore, acidosis is not evident until the renal failure stage and is only mild until uremia develops. The acidosis is, of course, metabolic and there is an increased anion gap due to the accumulation of anionic, conjugate bases normally excreted with the ammonium ion.
Phosphate excretion is regulated by the extent of tubular reabsorption from the filtrate which is controlled by parathyroid hormone. Normally, about 80-90% of filtered phosphate is reabsorbed with a normal positive calcium balance and basal levels of parathyroid hormone. Secondary hyperparathyroidism, which develops during the course of chronic renal disease from a negative calcium balance due to active vitamin D deficiency, diminishes phosphate reabsorption to a minimum of about 30% (a three-fold increase in excretion rate) so that hyperphosphatemia does not develop until the renal failure stage.
Urate and other organic anions are excreted primarily by active secretion for which there is 3 - 5 times the capacity normally required. Hyperuricemia does not develop, therefore, until the renal failure stage and worsens during uremia. The increase in other unmeasured anions is responsible for the increased anionic gap associated with the metabolic acidosis of severe renal disease.
____________________________________________________________ D. Deficient Elaboration of Active Vitamin D and Secondary HyperParathyroidism
Intestinal absorption of calcium, and of phosphate to a lesser extent, is regulated by circulating vitamin D3 concentrations. Active vitamin D (vitamin D3 or dihydroxy cholecalciferol) is elaborated exclusively by the kidneys. The elaboration rate of active vitamin D by individual nephrons is stimulated 3 - 5 fold by increased concentrations of parathyroid hormone. Adequate vitamin D3 concentrations can be maintained until the renal failure stage, after which intestinal absorption of calcium becomes deficient. Calcium deficiency then stimulates elaboration of parathyroid hormone the effects of which are to attempt to maintain normal serum concentrations of calcium by increasing bone resorption and increasing renal reabsorption of calcium. Near normal serum calcium concentration is thus maintained at the expense of increased bone resorption. The consequent bone wasting (renal osteodystrophy) generally presents as a mild form of osteomalacia.
____________________________________________________________ -
characteristic features:
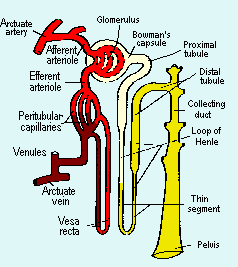 aaaNormal renal function represents the collective operation of a million or so nephrons. The function of each individual nephron requires the interdependence of four anatomic components:
aaaNormal renal function represents the collective operation of a million or so nephrons. The function of each individual nephron requires the interdependence of four anatomic components:
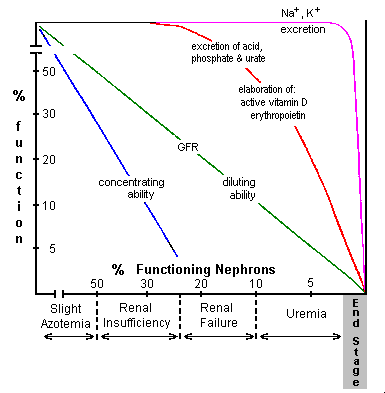 The manner in which different renal functions diminish as renal disease progresses and increasing numbers of nephrons are affected is illustrated in the figure to the right and is discussed below.
The manner in which different renal functions diminish as renal disease progresses and increasing numbers of nephrons are affected is illustrated in the figure to the right and is discussed below.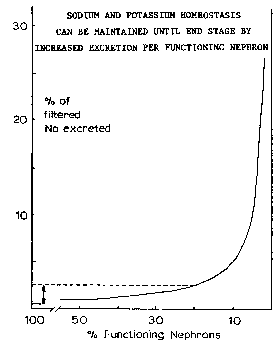 Normally, from 0.5 to 2.5% of filtered sodium and about 20% of filtered potassium is excreted. Sodium excretion is determined by the extent of aldosterone controlled reabsorption in the collecting tubules and in the absence of aldosterone, a maximum of about 2.5% of filtered sodium is normally excreted. Factors responsible for controlling excretion of potassium are less well established. Quite surprisingly, normal serum concentrations of sodium and potassium are maintained until near end stage. The fraction of filtered sodium and potassium excreted per functioning nephron increases in proportion to the decrease in number of functioning nephrons.
Normally, from 0.5 to 2.5% of filtered sodium and about 20% of filtered potassium is excreted. Sodium excretion is determined by the extent of aldosterone controlled reabsorption in the collecting tubules and in the absence of aldosterone, a maximum of about 2.5% of filtered sodium is normally excreted. Factors responsible for controlling excretion of potassium are less well established. Quite surprisingly, normal serum concentrations of sodium and potassium are maintained until near end stage. The fraction of filtered sodium and potassium excreted per functioning nephron increases in proportion to the decrease in number of functioning nephrons.